The main responsibility of all government drug regulatory agencies is to ensure that all products made available to its citizens conform to acceptable standards of quality, safety, and efficacy. Nubenco supports these regulations and assists various governments with the process, on behalf of our customers, in order to meet the health demands of their population. Nubenco has registered hundreds of medical devices and pharmaceutical products worldwide. Our highly experienced registration department deals with all matters relating to the registration of our products. For this purpose, Nubenco has its own professional staff, pharmaceutical chemists, lawyers, translators, engineers and other technical specialists from various countries, with the knowledge and experience needed to effectively and promptly register medical products. On some occasions, we will also contract external independent specialists. Our customers and suppliers rely on our experience and our knowledgeable staff to complete product registration effectively and efficiently until the product(s) are approved and ready for import, whether registration takes months or even years. Product registration procedures and requirements involve a highly detail oriented process that vary by country. However, our focus on the implementation of the most efficient registration processes has allowed us to expedite the amount of time it normally takes from submitting the registration application to when Nubenco products can be placed on the market.
The main responsibility of all government drug regulatory agencies is to ensure that all products made available to its citizens conform to acceptable standards of quality, safety, and efficacy. Nubenco supports these regulations and assists various governments with the process, on behalf of our customers, in order to meet the health demands of their population. Nubenco has registered hundreds of medical devices and pharmaceutical products worldwide. Our highly experienced registration department deals with all matters relating to the registration of our products. For this purpose, Nubenco has its own professional staff, pharmaceutical chemists, lawyers, translators, engineers and other technical specialists from various countries, with the knowledge and experience needed to effectively and promptly register medical products. On some occasions, we will also contract external independent specialists. Our customers and suppliers rely on our experience and our knowledgeable staff to complete product registration effectively and efficiently until the product(s) are approved and ready for import, whether registration takes months or even years. Product registration procedures and requirements involve a highly detail oriented process that vary by country. However, our focus on the implementation of the most efficient registration processes has allowed us to expedite the amount of time it normally takes from submitting the registration application to when Nubenco products can be placed on the market.
Registration Process
Registration submission
After all documents have been prepared and samples produced, complete registration files are submitted for approval.
It is vital to the success of not only the products being registered, but also all future products to be registered by Nubenco, that accuracy is absolute. We meticulously verify the accuracy of all documents and samples we submit.
Legal documents
Evidence that manufacturers and products are registered by a Stringent Regulatory Authority (SRA).
Sample preparation
Samples are required by most registering countries for testing in order to complete product registration.
Samples are produced according to the international standards required for product registration.
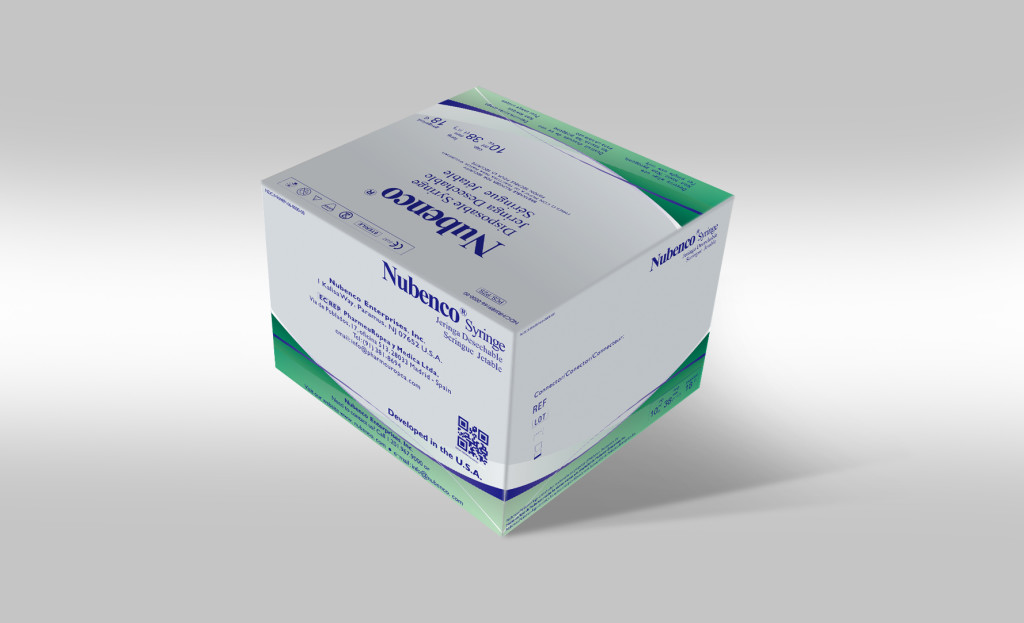
Packaging design
Packaging is designed according to the regulation requirements of each importing country. Superior modern packaging is one of the most important aspects in marketing our products.
Nubenco products are packaged with the best materials with the environment and product integrity in mind.
Agreements
All business relationships start with valid agreements in place, for distribution and manufacturing purposes.
Terms, conditions and specifications of Nubenco-client relationship are defined by both parties and remain confidential.
Dossier preparation
Nubenco begins preparing all necessary documents according to the importing country’s requirements.
Complete technical dossiers and other documents needed for registration are vital to register products in any country. Ministries of Health around the world know that when Nubenco registers its products, the process will be efficient and precise.